Interview series with Nicki Barker discussing Structured Light Plethysmography (SLP)
PneumaCare have been talking Structured Light Plethysmography (SLP) to a number of clinicians, all experts in their fields; discussing the clinical uses, benefits and future potential of SLP technology.
We have now posting chapters of our conversations with the Principal Clinical Researcher at Sheffield Children’s Hospital, Nicki Barker. You can watch the videos here. We hope you enjoy them.
Interview series with Professor Brendan Cooper discussing Structured Light Plethysmography (SLP)
PneumaCare have been talking Structured Light Plethysmography (SLP) to a number of clinicians, all experts in their fields; discussing the clinical uses, benefits and future potential of SLP technology.
We have now posting chapters of our conversations with Consultant Clinical Scientist in Respiratory and Sleep Physiology, Professor Brendan Cooper. You can watch the videos here. We hope you enjoy them.
Interview series discussing Structured Light Plethysmography (SLP)
PneumaCare have been talking Structured Light Plethysmography (SLP) to a number of clinicians, all experts in their fields; discussing the clinical uses, benefits and future potential of SLP technology.
We will be posting chapters of our conversations on social media and our website. Watch this space, we hope you enjoy them.
PneumaCare SLP Technology in Holby City
Did you know our innovative Structured Light Plethysmography (SLP) Technology appeared in an episode of Holby City, or that our imaging technology was originally developed for the movies? You can watch episode 18 of series 23 here.
You can see our technology approximately 11min 30sec into the episode. #SLP#HolbyCity



PneumaCare® create groundbreaking Normative Value Calculator for tidal breathing
PneumaCare® Limited, a Cambridge (UK) based medical device company are pleased to announce today, the publication release of their research outcomes on Normative Values in Tidal Breathing, measured by using their patented technology SLP (Structured Light Plethysmography). The study has been researched and written by leading clinicians over the past three years, establishing PneumaCare® as SLP pioneers in the field.
Tidal breathing measurements can be used to identify changes in respiratory status. Structured light plethysmography (SLP) is a non-contact, non-aerosol generating tidal breathing measurement technique. Lack of reference equations for SLP parameters makes clinical decision-making difficult.
PneumaCare have successfully developed a set of growth-adjusted reference equations for seven clinically pertinent parameters of respiratory rate (fR), inspiratory time (tI), expiratory time (tE), duty cycle (tI/total breath time), phase (thoraco-abdominal asynchrony (TAA)), relative thoracic contribution (RTC) and tidal inspiratory/expiratory flow at 50% volume (IE50).
SLP data from clinical and research measurements were collected from multiple sites (Queen Elizabeth (QE) Hospital, Birmingham, UK; Addenbrooke's Hospital, Cambridge University Hospitals NHS Foundation Trust, Cambridge, UK; University Hospital North Midlands (UHNM), Stoke-on-Trent, UK) were collated retrospectively.
This study has resulted in the production of an SLP normative value calculator developed to facilitate calculation of reference ranges for the above studied parameters. The calculator is colour coded to simulate a “traffic light” approach. In addition, it is possible to manually input observed values for each parameter and automatically obtain their corresponding z-scores.
PneumaCare’s CEO Bart Lewin, states, “The goal of this research was to provide an invaluable resource that will guide clinicians in their assessment, in turn optimize the treatment pathways for patients. PneumaCare has been successful in developing these reference ranges for seven key measures. Ensuring that clinicians and researchers can identify tidal breathing patterns in disease and better understand and interpret SLP and tidal breathing data.
This is an exciting time for PneumaCare®, the release of the Normative Value Research, combined with the launch of a pivotal COPD clinical trial could establish our SLP technology as a powerful yet diagnostic procedure with significant advantages over traditional methods”.
To download the white paper please visit https://openres.ersjournals.com/content/erjor/7/2/00050-2021.full.pdf
To learn more about SLP and the Normative Value Calculator contact us https://pneumacare.co.uk/contact/
Breaking new ground, PneumaCare® Ltd are at the forefront in changing the face of assessment in the respiratory field advancing non-contact, non-aerosol generating tidal breathing assessments. Using Thora-3Di®, patients can be assessed while breathing naturally and without contact.
For further information about the company and how our technology could support your organisation please visit www.pneumacare.com or contact us customerservice@pneumacare.com
PneumaCare® announce the launch of their COPD Clinical Study
PneumaCare® Ltd are excited to announce the launch of their COPD (COPD Chronic Obstructive Pulmonary Disease) clinical study. The study is a nationwide, comparative, multi-centered study designed to validate PneumaCare’s unique patented SLP based technology (Structured Light Plethysmography) against the current gold standard of care – spirometry.
The company’s mission is to provide a zero-contact, non-aerosol generating technology to actively assess lung function and to create diagnostic pathways in order to establish an enhanced standard of care. This pivotal study will focus on two objectives:
- Firstly, to determine SLP parameters in patients diagnosed with COPD and compare them with the results from the SLP normative values and the data collated from healthy smokers.
- Secondly, to validate SLP parameters for diagnosing patients diagnosed or suspected with COPD in comparison with spirometry results.
In the first stage of the study, PneumaCare will develop algorithms and parameters to predict FEV1 (the maximum amount of air forcefully exhaled in one second) and the final set of parameters will be validated in the second stage.
PneumaCare’s CEO Bart Lewin states,” If favorable results are achieved from the planned study, we envisage this will be an exciting time for the company, as it will further demonstrate that our SLP technology can be fully integrated into the UK’s health care system.
PneumaCare’s ambitious plan through this study is to establish an SLP based new standard of care to assist clinicians in the diagnosis of COPD. Specifically, to provide optimal algorithms to support a clear pathway to diagnosis.”
The first part of the study was initiated in May 2021, in Liverpool, one of the 6 investigational sites nationwide. A total of 500 subjects will be enrolled over the two-stage study. The study is expected to complete at the end of 2022. Please visit ClinicalTrial.GOV website for more information.
Breaking new ground, PneumaCare® Ltd are at the forefront in changing the face of assessment in the respiratory field advancing non-contact, non-aerosol generating tidal breathing assessments. Using Thora-3Di®, patients can be assessed while breathing naturally and without contact.
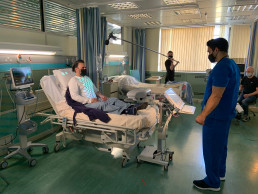
Thora-3Di® and PneumaView™ are already being used in a range of hospital environments from paediatric through to surgical assessment and adult intensive care. Further applications of PneumaCare’s proprietary Structured Light Plethysmography technology, currently in development with academics and clinicians, include dysfunctional breathing & COVID-19, pre & post-surgery applications.
For further information about the company and how our technology could support your organisation please visit www.pneumacare.com or contact us customerservice@pneumacare.com
PneumaCare® unveil a company rebrand in preparation for exciting 2021 clinical trial launch
PneumaCare® Limited, a Cambridge based medical device company, today unveiled a major rebrand in the forms of a new logo, colour scheme, and a major upgrade to the website. This change in identity reflects the recent evolution of the company. PneumaCare® is preparing to begin two major clinical trials in the areas of using our technology to diagnose chronic obstructive pulmonary disease (COPD) and dysfunctional breathing (DB) in 2021.

Bart Lewin, CEO of PneumaCare® states, “PneumaCare® is at an exciting crossroads. We are embarking on a pivotal COPD clinical trial this year that could establish our SLP technology as a powerful yet gentle diagnostic procedure with significant advantages over traditional methods.
Furthermore, in 2021 we will see the launch of our dysfunctional breathing study, which when combined with the COPD clinical trial, has the promise to establish PneumaCare® as a leader in breathing assessment.
The global pandemic has changed the face of healthcare; in many cases requiring new diagnostic treatment pathways for care providers and patients. Our system, the Thora-3Di®, provides a non-contact, non-invasive and more importantly, a non-aerosol generating lung function assessment procedure.”
Breaking new ground, PneumaCare® Ltd are at the forefront in changing the face of assessment in the respiratory field advancing non-contact, non-aerosol generating tidal breathing assessments. Using Thora-3Di®, patients can be assessed while breathing naturally and without contact.
For further information about the company and how our technology could support your organisation please visit www.pneumacare.com or contact us customerservice@pneumacare.com
Feasibility of Structured Light Plethysmography (SLP) in patients with Coronavirus disease 2019 (COVID-19)
The clinical manifestations of COVID-19 range from mild upper respiratory tract illnesses to progressive severe pneumonia, acute respiratory distress syndrome, multi-organ failure, and death. Measures to control the impact of the virus have affected job security, social contact and challenged health services, medical practices and policies. The health service has been radically mobilised to respond to the acute needs of patients infected with the virus at the same time as delivering scaled-back non-COVID-19 healthcare. In many surgical specialties, the management of perioperative patients has changed, with greater focus on remote triage through virtual consultations. However, the pre-operative evaluation of surgical candidates must happen at the clinic. One important aspect of pre-operative evaluation prior to thoracic surgery is pulmonary function testing (PFT), traditionally measured by conventional spirometry.
Understandably, concern has been raised that PFT represents a potential avenue for increased COVID-19 transmission due to increased viral dissemination. The healthcare sector is calling upon novel technology to replace PFT with a non-aerosol-generating alternative.
Structured Light Plethysmography (SLP) (PneumaCare Thora-3Di systems) has been proposed as a novel, non-contact, non-invasive method of assessing lung function. SLP offers real-time regional respiratory function via movement of the chest wall. This detailed information is translated into quantifiable pulmonary function outputs. SLP data may also be used to optimize non-invasive ventilatory settings. SLP has been able to successfully differentiate between the breathing patterns of healthy patients and those with COPD by mapping the thoracoabdominal displacement rate and accurately estimating inspiratory and expiratory flow (1). The ability to continuously measure these parameters may contribute to the safe weaning of patients from ventilatory support. Additionally, we hypothesise that SLP technology will allow clinicians to obtain measurements of breathing patterns that are closer to ecological conditions than those derived from spirometry measurements.
Our centre is currently trialling SLP in the work-up of pre-operative cardiothoracic patients. We feel it is intuitive to use and, therefore, does not require trained technicians, resulting in increased patient throughput and lower costs to the department. Importantly, SLP offers clinicians continuous measurement of mechanical chest wall displacement, a surrogate marker for fatigability and neuromuscular strength.
For the aforementioned reasons, we feel that SLP is a viable alternative to spirometry, especially in the current pandemic. Spirometry remains the gold-standard PFT, however, and the field would certainly benefit from studies evaluating the sensitivity, specificity and clinical validity of SLP in impairment detection, against gold-standard PFT.
Authors:
Natalie Simon M.Sc. MBBS
Academic Foundation Doctor, Cambridge University NHS Foundation Trust
Manish Soni, MBBS
Specialist Registrar
Bart’s Heart Centre St Bartholomew's Hospital
Trudy Kolvekar, MSc BSc
Bart’s Heart Centre St Bartholomew's Hospital
Amir Khosravi MD MRCS FRCSCTh
Senior Specialist Registrar Bart’s Heart Centre St Bartholomew's Hospital
Shyam Kolvekar MBBS, MS, MCh, FRCPS, FRCS, FRCSCTh
Consultant Cardiothoracic Surgeon Lead, Chest Wall Surgery Bart’s Heart Centre St Bartholomew's Hospital Hon. Associate Professor, University College London. West Smithfield London EC1A 7BE
PA: Rukia Khanom T:02034658689 rukia.khanom@nhs.net s.kolvekar@ucl.ac.uk
References:
- Motamedi-Fakhr S, Iles R, Barney A, De Boer W, Conlon J, Khalid A, Wilson RC. Evaluation of the agreement of tidal breathing parameters measures simultaneously using pneumotachography and structured light plethysmography. Physiological Reports. 2017;5(3): e13124 10.14814/phy2.13124
Lung function testing in the COVID-19 endemic
The COVID-19 pandemic has presented considerable challenges to global health services and dictates almost every aspect of medical practice and policy. Across Europe, a surge phase in acute caseload, led to a sudden curtailment of non-COVID-19 medical care, with immediate implications for routine diagnostic and surveillance investigations.
Pneumacare Launches SLP in Egypt
Pneumacare in conjunction with our new partner Global Health Care in Egypt present SLP technology to staff at Cairo Children's Hospital.